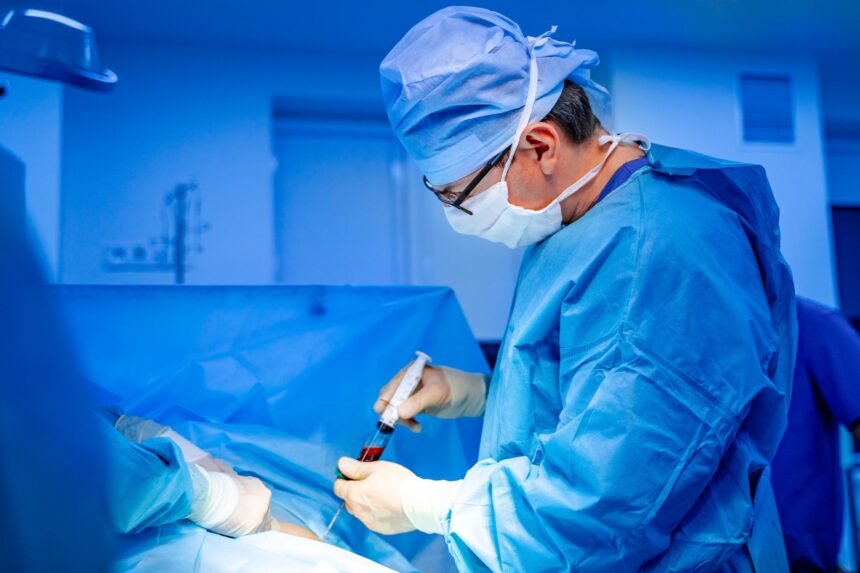
That appeared like excellent news: years after it grew to become obtainable in different nations, it was highly effective. Cell remedy for sort 1 diabetes Accepted within the US in June. It’s provided That is the primary methodology during which American sufferers obtain transplantation of insulin-producing islet cells exterior the boundaries of medical trials.
Nevertheless, the consultants who helped invent and develop islet transplant surgical procedure aren’t celebrating it. As an alternative, they criticize the U.S. Meals and Drug Administration (FDA) for selecting to control transplanted islet cells as medication somewhat than organs. Consequently, a single non-public firm will turn out to be the island’s solely permitted American provider for transplants.
“I do not perceive what the rationale is.” Camilo Ricordi, Marylandhonorary director of the Diabetes Institute relating to the choice to control organs as medication somewhat than organs. “There is no such thing as a scientific foundation for that.”
Piotr Witkowski, MD, PhD“There’s one voice within the transplant group. Nobody who understands transplant laws helps this,” mentioned Uchicago’s director of Pancreatic and Islet Transplant Program.
On daily basis in feedback on diabetes, the FDA stood behind the choice to approve remedy, however the island had not addressed the larger query of whether or not it must be regulated as a drug. Celltrans, an FDA-approved enterprise for distributing islands for porting, didn’t reply to a number of requests for remark.
Islet cell transplantation
Islet cell transplantation is a classy remedy for sort 1 diabetes. Merely put, docs take donor islets of the donor (cluster of pancreatic cells containing insulin-producing beta cells) and inject them into the diabetic sufferers, normally the liver. If profitable, the affected person can both fully discontinue insulin use or considerably scale back their reliance on insulin.
Some transplant recipients Insulin remedy has not been current for a few years Following the process, anti-rejection medication carrying their very own critical danger are required to guard new cells from the physique’s immune system.
The experiment is underway Assessing using laboratory-grown islet cellsNevertheless, at this time, these transplanted islet cells should be collected from the pancreas of deceased organ donors. Such donor cells are missing, limiting the variety of surgical procedures that may be carried out. Nevertheless, for sufferers with disastrous wants, similar to excessive glucose administration challenges and sufferers with out hypoglycemia, islet transplants is usually a lifesaver.
Regulation confusion
Shortly after the primary profitable islet transplantation in 1993, the FDA introduced that it might deal with the implanted islets as in the event that they had been. drugs Not an organ or subpart of an organ. This determination has confused consultants. “We’re the one nation that imposes this sort of regulation,” says Dr. Ricordi, who developed it in 1988. Islet cell separation expertise This makes porting potential.
The issue is that the organ donor’s physique island could not be capable to meet standards for accuracy and consistency of the drug elements. Like different organs, islet cells can’t precisely assess pre-transplant infertility, purity, or efficacy. Even when potential, the nonprofit analysis hospitals that developed the remedy don’t have the sources to fulfill the FDA’s expectations.
“Tutorial establishments haven’t been in a position to make investments thousands and thousands of {dollars} in jobs. bla (Software for a Biology License),” says Ricordi.
Over time, Dr. Witkovsky, Ricordi and plenty of of their colleagues marketing campaign It was named “Isle of Our Cooperation” to vary the FDA’s considering. “We have now informed the FDA that the island has been permitted as a drug and affected person security is in danger by for-profit organizations alone. The island must be regulated like another organ for transplant,” Witkowski says.
Universities and nonprofit hospitals couldn’t justify the prices, however the biotechnology firm named Cell transformer We raised sufficient funds to leap over the FDA hoops. Nevertheless, it was initially unclear whether or not Celtran might overcome the inevitable issues of consistency distinctive to human organs.
Between Listening to in April 2021the FDA Advisory Panel evaluated the outcomes of Celltrans medical trials. Witkowski mentioned the presentation confirmed what porting consultants already know. you may’t Meet the FDA’s said requirements for drug manufacturing. Naturally, the FDA discovered no correlation between the standard measures of islands and the medical efficacy measures.
“The FDA has clearly outlined why Celltrans failed,” claims Ricordi. Nonetheless, nearly all of impartial consultants on the panel agreed that remedy has a “total helpful danger profile for some sufferers with sort 1 diabetes.”
Panel approval didn’t shortly observe Sertran’s remedy in direction of approval. This process sat within the regulated limbo for about two years.
Stunning approval
Witkowski felt just like the island for US collaboration had lastly reached someplace in June. Senator Mikeley (R-UTAH) wrote legislative efforts to amend laws and pave the way in which for reliable islet transplants in America. Island act He promised to “transferring the island right into a extra acceptable regulatory framework.” However Witkowsky’s optimism was short-lived.
Per week after Senator Lee introduced the invoice, the FDA introduced it had permitted Celtlan’s lifeless island. The sauce for the brand new island will likely be named Doniclecel (Lantidra). The island cooperation for us responded with doubt, Assertion The transfer “considerably difficult the trail to passing the Island Act and the trail to implementing vital regulatory updates.”
Celltrans needed to spend thousands and thousands to file a profitable BLA, however critics argue that the enterprise did not truly develop something new. a 2021 Letter in Worldwide transplantation “It is only a new title for allografting of islets,” Lantidra mentioned.
Witkowski agrees: “They invented nothing. They did not change something. It is an unchanged human organ, however they name it a drug and promote it as a drug.”
Lantidra is presently the one FDA permitted supply of islets for transplantation for the remedy of sort 1 diabetes.
Islet Transplantation and Dangers
Islet cell transplantation has nice potential to deal with sort 1 diabetes. In a very powerful trial of Lantidra, performed at an instructional transplant middle following all identified protocols, 30% of members achieved insulin independence in at the very least 5 years.
Nevertheless, islet transplantation primarily includes unwanted effects profiles with potent immunosuppressive medication wanted to guard new cells. A whopping 87% of Lantidra examination members skilled at the very least one “extreme” response, and 27% skilled at the very least one Threatening life Unwanted side effects. Amongst 30 sufferers, there have been 211 an infection instances. The an infection precipitated sepsis, multi-organ failure, and one other topic died when he developed a life-threatening liver laceration.
Witkowski says, “This wasn’t a shock to us.” The dangers inherent in islet transplantation are vital. That is a part of the explanation why this process is restricted to sufferers with the deepest glucose administration challenges.
Nevertheless, Witkowski worries that Lantidra’s danger profile may very well be even worse in the actual world. As a result of distributing it as a drug overturns the chain of duty that helps hold organ transplants secure.
Historically, islet transplant surgeons take possession of all facets of the surgical procedure. Repeatedly monitor organ choice and analysis, surgical operations, and affected person monitoring.
“I take duty for doing every little thing,” Witkowski says. “I select every little thing with the donor. If there may be something flawed, it’s in me and my transplant middle and I must disclose the outcomes.”
Nevertheless, by distributing Lantidra as a drug, “surgeons lose management of the product. There is no such thing as a selection. They must take what’s given.”
Celltrans can select to promote Lantidra solely to non-profit transplant facilities and put it within the palms of essentially the most certified surgeons. However it could possibly additionally ignore the standard community of transplant facilities and promote the island to non-public services. Witkowski is especially involved about this latter risk. In non-public clinics the place there isn’t any requirement to reveal outcomes, there could also be “no duty…no later supervision.”
“They will select to do it the suitable approach, however they don’t seem to be obligated to do it the suitable approach.”
Moral issues
Authors of 2021 Worldwide transplantation The letter, a constellation of European endocrinologists, immunologists and transplant surgeons, condemned the moral implications of approving Lantidra as a drug.
Granting non-public business enterprises advertising rights to isolate related islands can foreshadow the commercialization of human organs and their subparts.
Specialists outlined different points. He argued that Lantidra’s approval would doubtless block competitors and restrict remedy to the wealthiest individuals of his sufferers.
Witkowski shares the identical issues. In keeping with him, the ready checklist for organ transplants is United Community for Organ Sharing (unos), a nonprofit group dedicated to equitable distribution of organs based mostly on affected person wants. However, as a non-public firm, Celtran “can select who they wish to give it.” Island is eligible for insurance coverage reimbursement, however the enterprise could have a robust incentive to connect a excessive value to Lantidra and out of attain of some sufferers in want.
Celtran was granted seven years of exclusiveness beneath the Orphan Drug Act, however enterprise Formally He has pledged to desert exclusivity and permit different firms to submit BLAs and take part as an permitted island provider. Nevertheless, Witkowski identified that Celltrans can simply revoke its exclusiveness.
“It’s totally unlucky.”
The approval of Lantidra as a drug seems to fly within the face of a practice of non-profit cooperation that helped develop the island transplant within the first place.
When Ricordi invented the approach for islet cells, a method that Celtran wanted for Lantidra, he shared it freely.
Ricordi defined that he was impressed by the scientists who found insulin, saying he needed it to sound inevitable. Nobel Prize winner Frederick Bunting and his colleagues The insulin patents had been offered for $1.00 every Within the Nineteen Twenties, life-saving medicines had been distributed as shortly and affordably as potential.
Brutal irony is that Ricordi’s innovation is now basically reserved in the US for the unique use of unrelated, business companies.
“To see numerous effort to maintain nonprofits and see every little thing swept into business entities attributable to this outdated FDA laws…it is a very disappointing factor. I am proud of the Sertran. I am not criticising them. They play with the principles.
Witkowski will not be positive what is going to occur subsequent. He hopes Senator Lee and different lawmakers who’ve pledged to assist the island’s legislation is not going to retreat.
“However I do not know what is going to occur. I do not suppose they had been anticipating this response from the FDA. I am ready for them to surrender or allow them to know if they will proceed combating.”
This legislation requires bipartisan cooperation to cross the legislation. It’s missing in our damaged political atmosphere.
Witkowski mentioned, “If this island passes by means of its actions, it turns into a nationwide useful resource, similar to all different organs, and is protected by legislation.”