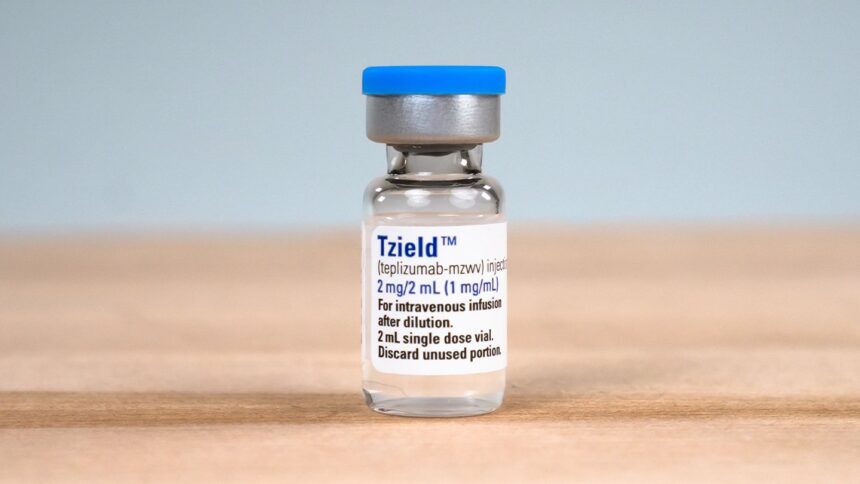
For the primary time, the US Meals and Drug Administration (FDA) has permitted a drug that might delay the onset of kind 1 diabetes.
Teprizumab-MZWV (Tzield), an intravenous drug, is meant for adults and kids aged 8 and older who’re at excessive danger for kind 1 diabetes however haven’t but been identified with a situation and should not reliant on insulin photographs. Tzield is in a brand new household of medication that gradual the immune system’s assault on pancreatic insulin-producing cells, the underlying reason for kind 1 diabetes.
“Software for remedy in in the present day’s class provides vital new therapy choices to sufferers at particular danger,” stated John Schalletz, MD, director of diabetes, lipid problems and weight problems on the FDA’s Heart for Drug Analysis and Analysis. “The opportunity of delaying the scientific prognosis of kind 1 diabetes may present sufferers with months to years with out the burden of illness.”
In kind 1 diabetes, the pancreas stops producing adequate insulin. Insulin is a hormone that helps the physique convert sugar into meals it eats for vitality. With out insulin, sugar accumulates within the bloodstream. This can be a situation that with out therapy can result in severe issues corresponding to coronary heart illness, kidney failure, amputation, and blindness. Folks with kind 1 diabetes want lifelong therapy with insulin photographs or insulin pumps.
In line with the American Diabetes Affiliation, most individuals who develop kind 1 diabetes have inherited the chance components of their dad and mom. Folks with kind 1 diabetes dad and mom or siblings can take blood exams to see if their our bodies are starting to assault insulin-producing cells within the pancreas. As these assaults progress, individuals nearly at all times develop kind 1 diabetes and require insulin photographs, however the tempo of this development is totally different.
Tzield can gradual the development of individuals referred to as stage 2 illnesses. In line with Emory College, this isn’t a full-scale kind 1 diabetes that also requires therapy with insulin, however individuals with stage 2 illness have broken insulin-producing cells to their pancreas and irregular blood glucose ranges.
The FDA permitted Tzield primarily based on a scientific trial in 76 sufferers with two phases of sickness. On this trial, scientists randomly assigned individuals to obtain a every day 14-day infusion of a placebo.
After a median follow-up of 51 months, 45% of sufferers within the Tzield group developed kind 1 diabetes, in comparison with 72% with placebo.
The typical development time to kind 1 diabetes was additionally twice as lengthy in Tzield. The placebo group was 50 months in comparison with 25 months.
Stopping this prognosis for a number of years, particularly for youngsters at excessive danger of kind 1 diabetes, is vital to offer them extra mature and time to navigate every day life on this situation.
“The delay within the onset of kind 1 diabetes is a press release after the FDA permitted Tzield, Dr. Aaron Kowalski, CEO of the Juvenile Diabetes Analysis Basis, stated:
“It can relieve them of the fixed burden and stress of blood glucose monitoring and insulin administration,” stated Dr. Kowalski. “It can free them from fear and worry about short- and long-term issues, whereas additionally giving them the chance to study extra about illness administration.”
The most typical uncomfortable side effects of Tzield embody decrease leukocyte ranges, rashes and complications, the FDA says. Extra severe, extra severe uncomfortable side effects embody infections, interference with routine childhood vaccinations, and what is called cytokine launch syndrome.
In July 2021, the FDA delayed approval, with regulators asking for particulars on how the physique responds to medicine. As developer Bio stated on the time, it was once more postponed to June 2022 because the FDA prolonged its medical assessment interval.